Optimize Technical Documentation for Life Sciences, Healthcare & Pharmaceutical
Life Sciences, healthcare and pharmaceutical companies are streamlining the creation and delivery for medical device user guides, instructional manuals, technical brochures, online Help and more.
By utilizing technical documentation software for life sciences, healthcare, or pharmaceutical companies, organizations can efficiently produce and manage complex materials.

Maintain Strict Documentation Standards in Highly Regulated Industries
- Accelerate documentation deliverables with agile content development lifecycles with single-sourcing
- Deliver integrated content for websites, PDFs, tablets and mobile devices all from a single project
- Reduce production time with improved workflows and a streamlined content review process enabling faster delivery of medical technical writing and technical concepts
- Customize content for different roles and audiences using variables and condition tags
- Streamline the translation process and cut localization costs with integrated Translation Memory and project management
- Create more meaningful and interactive user experience with image callouts and labels for equipment, procedures and more, helping to simplify complex technical concepts for end-users
Doing all of our technical content in Flare has enabled us to do more than we could before, simply because everything is together, done the same way, and can be produced in the same target. Flare has effectively doubled our productivity.

Thad Miller Technical Content Director, LI-COR Biosciences


Largest Healthcare Services Company in the United States Switches to MadCap Flare for Agile Delivery of Online Help and Documentation Supporting Multiple Product Versions
Agile Delivery of Online Help and Documentation
Flare’s single-sourcing capabilities help us streamline our processes to rapidly deliver the information our customers need for any new product releases.
Pat Holmes-Clark | Team Leader and Specialist Technical Writer, McKesson Health Solutions
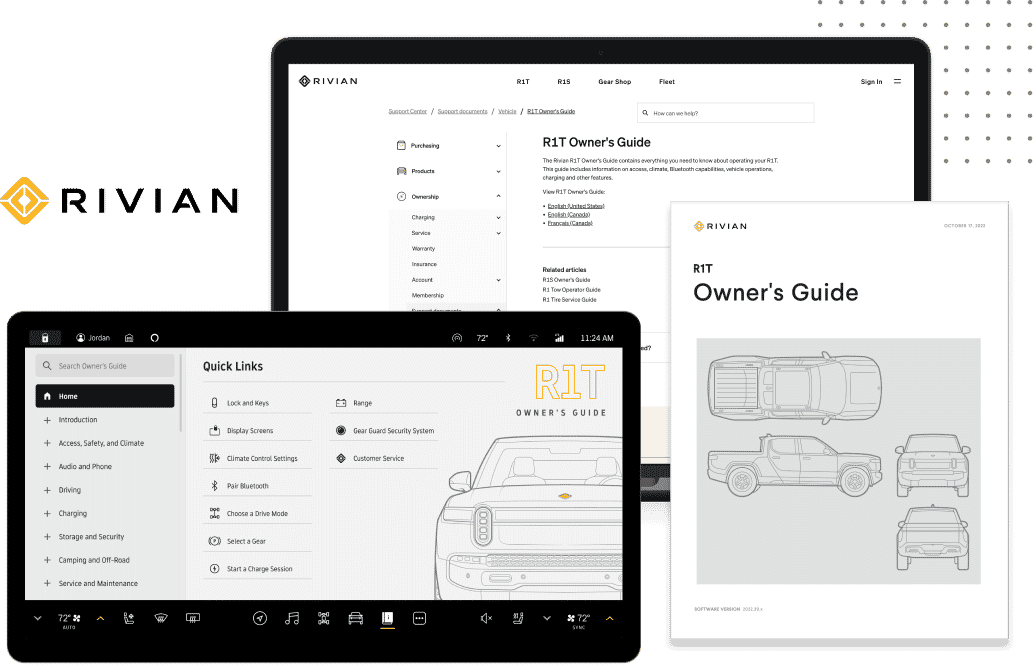
Leading Life Sciences Company, Illumina, Uses MadCap Software to Optimize Version Control and Cut Project Time Up to 80 Percent
Reduced Project Time From Six Weeks to One Week
athenahealth Uses MadCap Flare, Contributor and Feedback to Deliver State-of-the-Art Documentation and Online Help for its Cloud-based Physician Business Services
Doubled Productivity for Technical Writers and SMEs
It was exciting to see how quickly we were able to get the mobile documentation published with Flare.

Lynn M. Carrier Assoc. Director, Content Process and Technology, Illumina
Leading Life Sciences, Healthcare & Pharmaceutical Companies Trust MadCap Software

Frequently Asked Questions
What types of technical documentation are required for life sciences, healthcare, and pharmaceutical companies?
Life sciences, healthcare, and pharmaceutical companies typically produce user manuals, medical device documentation, instructional guides, technical brochures, and API documentation. MadCap's tools support the efficient creation and management of these complex materials.
How does MadCap ensure compliance with industry standards in healthcare and pharmaceutical documentation?
MadCap ensures compliance with healthcare and pharmaceutical regulations by providing structured workflows, version control, and audit trails. These features help meet strict regulatory standards, such as FDA, HIPAA, and other industry-specific guidelines while ensuring high-quality and compliant documentation.
How can MadCap streamline the translation and localization process for healthcare and pharmaceutical companies?
MadCap integrates localization and translation services to help life sciences, healthcare, and pharmaceutical companies reduce localization costs and accelerate the translation process, ensuring consistency and accuracy across multiple languages.
How does MadCap help manage large-scale documentation projects in the life sciences and healthcare industries?
With features like DITA support and single-source publishing, MadCap simplifies large-scale documentation management, enabling efficient handling of complex, multi-format documentation projects for various stakeholders.
What security features does MadCap provide for sensitive healthcare and pharmaceutical documentation?
MadCap includes robust security features such as SSL encryption, user authentication, role-based access permissions, content management security, and version control, ensuring sensitive healthcare and pharmaceutical documentation is securely managed and fully compliant with industry regulations.
How can MadCap’s AI capabilities enhance documentation workflows in life sciences and healthcare?
MadCap's AI tools help automate content creation, streamline the organization, and optimize document structure, allowing teams to work smarter and faster. AI assists in suggesting content, improving the accuracy of regulatory documentation, and accelerating the translation process, ensuring consistency and quality across all documentation outputs.
Empower Your Technical Documentation with MadCap - Life Sciences & Pharmaceutical
MadCap Software’s suite of tools enhances every aspect of technical documentation and content management for life sciences and pharmaceutical companies. MadCap Flare, a powerful technical writing software, supports the creation of single-source documentation, including user manuals, product documentation, and API documentation. With MadCap Flare Online, a cloud based content management tool, teams can collaborate seamlessly with version control, documentation management, and efficient review workflows.
Tailored for the healthcare industry, MadCap provides advanced technical authoring tools for healthcare companies, making it a leading medical documentation software for ensuring compliance with industry standards. It enables teams to efficiently create medical technical writing and technical concepts while ensuring compliance with industry standards. Paired with MadCap IXIA CCMS, a DITA technical writing software, that simplifies large-scale documentation projects, delivering high-quality, reliable content that meets the needs of both technical writers and developers. Together, these tools streamline the documentation process, ensuring accuracy, efficiency, and compliance while empowering teams to produce consistent, high-quality content across all stages of the project.
Learning Resources Available

Videos & Tutorials
Learn how MadCap Software streamlines content delivery with advanced topic-based authoring, single-sourcing and multi-channel publishing.
Watch Tutorials, Demos and See What's New
Watch Videos
Customer Showcase
Get inspired with the latest collection of documentation websites, user manuals, support portals, knowledge bases and more.
Browse Customer Showcase Samples
Browse Top Industries
Product Overview
Learn how our industry leading on-premises and cloud-based solutions can drive more value for your customers – and your business.
Learn More About Our Solutions
Watch VideosIt’s easy to get started with MadCap Software
Get in touch with a product specialist to schedule a personalized demo.